Tumor Mutational Burden Market to Reach USD 6.76 Billion by 2036 as Genomic Metrics Reshape Immunotherapy Decisions
TMB testing demand rises with immunotherapy adoption, driven by NGS-based profiling, standardized scoring, and selective clinical use in oncology care.
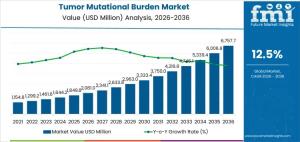
NEWARK, DE, UNITED STATES, January 19, 2026 /EINPresswire.com/ -- The global tumor mutational burden market is forecast to reach a value of USD 2,081.0 million in 2026 and expand to USD 6,757.7 million by 2036, reflecting a compound annual growth rate (CAGR) of 12.5%. Growth is driven by the deeper integration of genomic metrics into immunotherapy decision-making rather than broad-based diagnostic screening. TMB testing is increasingly used to guide immune checkpoint inhibitor prescribing, where mutation load influences patient eligibility, therapy sequencing, and combination strategies across multiple solid tumor types.
Market activity is concentrated in oncology centers and reference laboratories with established next-generation sequencing (NGS) and validated bioinformatics infrastructure. Cost structures remain higher than single-gene assays due to panel size, sequencing depth, data interpretation requirements, and quality assurance obligations. As a result, utilization remains selective and closely tied to defined clinical indications rather than routine population-wide testing.
Market Context: Why Tumor Mutational Burden Matters in Precision Oncology
Tumor mutational burden measures the number of somatic mutations per megabase of tumor DNA. Higher mutation loads are associated with increased neoantigen presentation, which may improve responsiveness to immune checkpoint inhibitors in cancers such as melanoma, lung cancer, and urothelial carcinoma. As oncology practices move beyond single-gene biomarkers, TMB has emerged as a quantitative tool to address response uncertainty and treatment cost exposure in immuno-oncology care.
Laboratories and pathologists are adopting TMB assays on NGS platforms to support clinical decision-making. Procurement teams evaluate assay performance, platform interoperability, turnaround time, and bioinformatics pipelines, as reproducible and harmonized TMB scoring is essential for consistent treatment selection and integration with electronic health records.
Key Market Metrics and Growth Outlook
• Market value (2026): USD 2,081.0 million
• Forecast value (2036): USD 6,757.7 million
• Forecast CAGR (2026–2036): 12.5%
• Leading offering by demand: Targeted NGS TMB panels
• Fastest-growing countries: India, Brazil, China, United States, Germany
Value expansion reflects guideline reinforcement, broader immunotherapy utilization, and continued reliance on mutation burden as a clinically relevant genomic metric rather than an increase in overall cancer incidence.
Offerings Analysis: Preference for Scalable and Actionable Solutions
Targeted NGS TMB panels account for 26.0% of market demand, balancing sequencing depth, cost control, and workflow integration. Central laboratory TMB testing services and liquid biopsy-based TMB assays each represent 20.0%, supporting standardized execution and minimally invasive sampling. Whole-exome sequencing holds an 18.0% share, primarily in research and comprehensive analysis contexts, while bioinformatics and interpretation software accounts for 16.0%, reflecting the importance of harmonized scoring and reporting.
Applications Driving Utilization
Immunotherapy patient selection represents the largest application segment at 34.0%, underscoring TMB’s role as a predictive biomarker for checkpoint inhibitor response. Comprehensive tumor profiling accounts for 26.0%, integrating TMB alongside actionable genomic alterations. Clinical trials and translational research represent 18.0%, followed by therapy response monitoring (12.0%) and population genomics programs (10.0%).
Testing Channels and Delivery Models
Reference laboratories lead with a 30.0% share due to centralized expertise and validated workflows. Hospital molecular pathology laboratories account for 24.0%, supporting integrated diagnostics within oncology care pathways. Academic cancer centers (18.0%), biopharma companies (16.0%), and contract research organizations (12.0%) further contribute to demand, particularly in drug development and clinical trials.
Global Demand Trends and Regional Growth
Global demand for TMB testing is expanding alongside increased immunotherapy adoption and broader access to NGS platforms. Growth rates vary by country, reflecting differences in reimbursement clarity, assay standardization, and diagnostic infrastructure:
• India: 15.0% CAGR, driven by private oncology network expansion and affordable sequencing
• Brazil: 14.5% CAGR, supported by immunotherapy uptake and judicial access pathways
• China: 14.0% CAGR, aligned with protocol-driven adoption in tertiary hospitals
• United States: 11.0% CAGR, reinforced by guideline recognition and payer coverage
• Germany: 10.9% CAGR, shaped by evidence-based reimbursement governance
Competitive Landscape and Industry Positioning
The competitive environment emphasizes assay reproducibility, regulatory alignment, and integration with immunotherapy decision pathways. Roche (Foundation Medicine) leads through clinically validated TMB assays embedded in comprehensive genomic profiling. Illumina, Thermo Fisher Scientific, Guardant Health, and QIAGEN support demand through sequencing platforms, decentralized laboratory solutions, blood-based TMB testing, and sample-to-insight workflows. Competitive differentiation centers on clinical validation depth, bioinformatics accuracy, and compatibility with tissue and liquid biopsy workflows.
Explore trends before investing – request a sample report today! https://www.futuremarketinsights.com/reports/sample/rep-gb-31385
Market Dynamics and Outlook
While demand continues to grow, scalability is moderated by methodological variability, reimbursement uncertainty, and the need for specialized data interpretation expertise. Tissue adequacy constraints and sensitivity limitations in low-shedding tumors further reinforce selective deployment. Long-term growth will depend on continued guideline support, assay harmonization, and the expanding role of TMB as a standardized quantitative biomarker in precision oncology.
Browse Related Insights
Tumor Endoprostheses Market: https://www.futuremarketinsights.com/reports/tumor-endoprostheses-market
Tumor Profiling Market Share Analysis: https://www.futuremarketinsights.com/reports/tumor-profiling-market-share-analysis
Tumor-sequencing Blood Testing Market: https://www.futuremarketinsights.com/reports/tumor-sequencing-blood-testing-market
Sudip Saha
Future Market Insights Inc.
+18455795705 ext.
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.